
Hetero Gets DCGI Nod To Launch Anti Hepatitis C Drug In India
Hetero, generic pharmaceuticals and anti-retroviral drug manufacturer is all set to launch the fixed-dose combination therapy ‘Ledipasvir-Sofosbuvir’ for the Indian patients. Hetero today announced that it is the first company in India to receive the Drug Controller General of India (DCGI) approval for the fixed-dose combination drug Ledipasvir-Sofosbuvir (90mg/400mg).
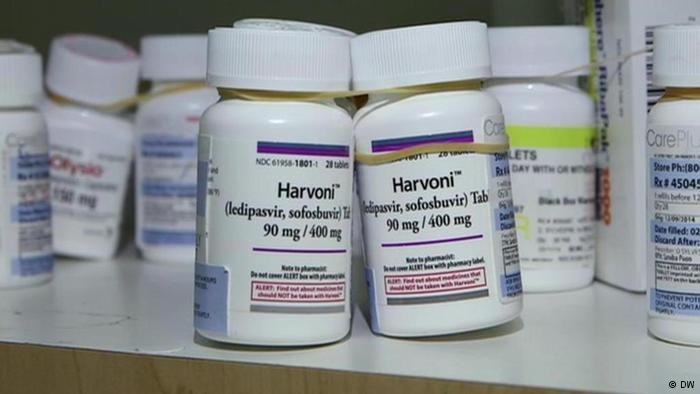
The product will be available under the brand name ‘Ledisof’ in India. The drug, Ledisof, is a generic version of Gilead Sciences’ brand Harvoni which is approved by the US FDA for the treatment of chronic hepatitis genotype 1 in naive and treatment experienced patients. Sofosbuvir in combination with ledipasvir has shown to have high cure rates of around 90 per cent.
“We have been the front-runners in launching the generic sofosbuvir in several countries. We are now happy to extend the fixed dose combination therapy Ledipasvir sofosbuvir (Ledisof) to Indian patients which is much more effective than Sofosbuvir,’’ BPS Reddy, Chairman and Managing Director of Hetero said, adding, “With the launch of Ledisof, we look forward in bringing a paradigm shift in hepatitis C management in the country.”
The Hyderabad-based Hetero had signed a non-exclusive licensing agreement with Gilead in September last year to manufacture and market the drug indicated for the treatment of chronic hepatitis C.
Hepatitis C is a growing health concern in many developing countries including India which is estimated to have about 12 to 18 million people infected with hepatitis C. Untreated chronic hepatitis C increases the risk of cirrhosis of liver, liver failure and hepatocellular carcinoma.